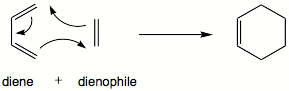Diels–Alder reaction

In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile (also spelled dieneophile), to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed cycloaddition with Woodward–Hoffmann symbol . It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reactions becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction. The reaction is an example of a concerted pericyclic reaction. It is believed to occur via a single, cyclic transition state, with no intermediates generated during the course of the reaction. As such, the Diels–Alder reaction is governed by orbital symmetry considerations: it is classified as a cycloaddition, indicating that it proceeds through the suprafacial/suprafacial interaction of a 4π electron system (the diene structure) with a 2π electron system (the dienophile structure), an interaction that leads to a transition state without an additional orbital symmetry-imposed energetic barrier and allows the Diels-Alder reaction to take place with relative ease. A consideration of the reactants' frontier molecular orbitals (FMO) makes plain why this is so. (The same conclusion can be drawn from an orbital correlation diagram or a Dewar-Zimmerman analysis.) For the more common 'normal' electron demand Diels–Alder reaction, the more important of the two HOMO/LUMO interactions is that between the electron-rich diene's ψ2 as the highest occupied molecular orbital (HOMO) with the electron-deficient dienophile's π* as the lowest unoccupied molecular orbital (LUMO). However, the HOMO–LUMO energy gap is close enough that the roles can be reversed by switching electronic effects of the substituents on the two components. In an inverse (reverse) electron-demand Diels–Alder reaction, electron-withdrawing substituents on the diene lower the energy of its empty ψ3 orbital and electron-donating substituents on the dienophile raise the energy of its filled π orbital sufficiently that the interaction between these two orbitals becomes the most energetically significant stabilizing orbital interaction. Regardless of which situation pertains, the HOMO and LUMO of the components are in phase and a bonding interaction results as can be seen in the diagram below. Since the reactants are in their ground state, the reaction is initiated thermally and does not require activation by light. The 'prevailing opinion' is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry. There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as dimethylformamide and ethylene glycol. and even in water. The reaction of cyclopentadiene and butenone for example is 700 times faster in water relative to 2,2,4-trimethylpentane as solvent. Several explanations for this effect have been proposed, such as an increase in effective concentration due to hydrophobic packing or hydrogen-bond stabilization of the transition state. The geometry of the diene and dienophile components each propagate into stereochemical details of the product. For intermolecular reactions especially, the preferred positional and stereochemical relationship of subtituents of the two components compared to each other are controlled by electronic effects. However, for intramolecular Diels–Alder cycloaddition reactions, the conformational stability of the structure the transition state can be an overwhelming influence. Frontier molecular orbital theory has also been used to explain the regioselectivity patterns observed in Diels–Alder reactions of substituted systems. Calculation of the energy and orbital coefficients of the components' frontier orbitals provides a picture that is in good accord with the more straightforward analysis of the substituents' resonance effects, as illustrated below. In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ortho and para positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the 'ortho' product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the 'para' product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.